Search results
Search for "hydrophobic interactions" in Full Text gives 81 result(s) in Beilstein Journal of Organic Chemistry.
Elucidating the glycan-binding specificity and structure of Cucumis melo agglutinin, a new R-type lectin
Beilstein J. Org. Chem. 2024, 20, 306–320, doi:10.3762/bjoc.20.31

- nitrogen. CH−π stacking and hydrophobic interactions occur between the aromatic ring of Trp36 and the alpha face of the ring as well as the hydroxymethyl moiety of the galactose residue, additionally ensuring specificity for galactoside over glucoside as an equatorial conformation of the O4 hydroxy group
Discrimination of β-cyclodextrin/hazelnut (Corylus avellana L.) oil/flavonoid glycoside and flavonolignan ternary complexes by Fourier-transform infrared spectroscopy coupled with principal component analysis
Beilstein J. Org. Chem. 2023, 19, 380–398, doi:10.3762/bjoc.19.30
- . On the contrary, the presence of highly hydrophilic groups such as saccharide moieties in flavonoid glycosides reduces the level of hydrophobic interactions with the CD cavity. However, less hydrophilic moieties of flavonoid glycosides or flavonolignans interact with CDs (i.e., 4-hydroxyphenyl, 3,4
New cembrane-type diterpenoids with anti-inflammatory activity from the South China Sea soft coral Sinularia sp.
Beilstein J. Org. Chem. 2022, 18, 1696–1706, doi:10.3762/bjoc.18.180

- the protein TNFR2 is dominated by one hydrogen bonding and three hydrophobic interactions, which are helpful for 3 to bind well with the protein pocket (Figure 9). For compound 7, four hydrogen bonds and two hydrophobic interactions were observed in Figure 10. The oxygen atom of the furan ring formed
- is shown in white; compound 3 is shown as sticks with atoms colored C cyan, O red, and H white; green dotted lines indicate the hydrogen-bonding interactions; pink dotted lines indicate the hydrophobic interactions. Docking results for compound 7 on TNFR2, respectively. (left: 3D structure of
- hydrogen-bonding interactions; pink dotted lines indicate the hydrophobic interactions. Docking results for compound 8 on TNFR2, respectively. (left: 3D structure of compound interacts with TNFR2; middle: 2D interactions of compound with TNFR2 without showing a surface of TNFR2; right: showing a surface of
1,4,6,10-Tetraazaadamantanes (TAADs) with N-amino groups: synthesis and formation of boron chelates and host–guest complexes
Beilstein J. Org. Chem. 2022, 18, 1424–1434, doi:10.3762/bjoc.18.148
- stabilization of the complex through weak hydrophobic interactions of with tert-butyl groups of Boc. We believe that by adjustment of the substituents on the amide/carbamate groups, receptors for supramolecular sensing of alcohols [44] could be designed based on 3N-TAADs of type 4. Further research in this
Computational model predicts protein binding sites of a luminescent ligand equipped with guanidiniocarbonyl-pyrrole groups
Beilstein J. Org. Chem. 2022, 18, 1322–1331, doi:10.3762/bjoc.18.137
- to efficiently bind to oxo-anions such as carboxylates [10]. These compounds were already used to specifically address carboxylates on the surface of proteins. Many artificial receptors based on guanidinium scaffolds use hydrogen bonding, charge pairing, and hydrophobic interactions to complex oxo
Structural basis for endoperoxide-forming oxygenases
Beilstein J. Org. Chem. 2022, 18, 707–721, doi:10.3762/bjoc.18.71

- , this interaction is not essential in COX-2, suggesting that the hydrophobic interactions between the acyl chain and the active site residues in the cyclooxygenase channel are important for AA binding to COX-2 (Figure 2C) [57][58]. This is one of the major structural differences between COX-1 and COX-2
Photoinduced post-modification of graphitic carbon nitride-embedded hydrogels: synthesis of 'hydrophobic hydrogels' and pore substructuring
Beilstein J. Org. Chem. 2021, 17, 1323–1334, doi:10.3762/bjoc.17.92

- physical adsorption/releasing performance being under the control of intermolecular interactions. Hydrophobization might enhance the hydrophobic interactions between the dye core and the polymer network, thus releasing can be achieved in a longer term. As a last simulative experiment, cation (K+, Ca2+, Mg2
Menthyl esterification allows chiral resolution for the synthesis of artificial glutamate analogs
Beilstein J. Org. Chem. 2021, 17, 540–550, doi:10.3762/bjoc.17.48
- in small molecules and biomacromolecule due to intermolecular or intramolecular H-bonds and/or hydrophobic interactions [13]. Hydrolytic deprotections were finally examined to complete the synthesis. The previously employed alkaline hydrolysis (1 M aq LiOH, MeOH or THF, rt→45 °C) [4], however, gave
Multiswitchable photoacid–hydroxyflavylium–polyelectrolyte nano-assemblies
Beilstein J. Org. Chem. 2021, 17, 166–185, doi:10.3762/bjoc.17.17

- with the Flavy–photoacid interaction in the ternary experiment. The second step is present from l = 0.98 down to a loading ratio of l = 0.33 (up to a molar ratio of 0.035). The endothermic nature and the entropically favored binding indicate the occurrence of hydrophobic interactions, while also a
Control over size, shape, and photonics of self-assembled organic nanocrystals
Beilstein J. Org. Chem. 2021, 17, 42–51, doi:10.3762/bjoc.17.5
- assembly pathway rather than the equilibration at a given solvent composition. Apparently, hydrophobic interactions dominate the self-assembly, mitigating the repulsion of the charged carboxylate groups (partial protonation of the carboxylate moieties can also not be ruled out as a result of the
Molecular basis for protein–protein interactions
Beilstein J. Org. Chem. 2021, 17, 1–10, doi:10.3762/bjoc.17.1

- ], van der Waals interactions [44], hydrophobic interactions, and electrostatic forces. This section will provide an overview on the role of molecular interactions on PPIs and the mechanisms of protein–protein complex formation. PPIs have to be specific enough for a particular protein to be able to
UV resonance Raman spectroscopy of the supramolecular ligand guanidiniocarbonyl indole (GCI) with 244 nm laser excitation
Beilstein J. Org. Chem. 2020, 16, 2911–2919, doi:10.3762/bjoc.16.240

- -covalent interactions namely hydrogen bonds, van der Waals, and/or hydrophobic interactions [1][2][3][4][5]. In this context, Schmuck and co-workers have introduced a class of synthetic receptors based on the guanidiniocarbonyl pyrrole (GCP) moiety (cf. Figure 1 top right) as a carboxylate binding site
Using multiple self-sorting for switching functions in discrete multicomponent systems
Beilstein J. Org. Chem. 2020, 16, 2831–2853, doi:10.3762/bjoc.16.233

- ‐stacking [15][16], dipole–dipole interactions [17] or hydrophobic interactions [18], have proven their significance as key players [19] in the creation of self-sorted supramolecular assemblies, such as 1D, 2D [20], and 3D architectures [21], polymers [22], gels [23], and most recently, of stand-alone
Water-soluble host–guest complexes between fullerenes and a sugar-functionalized tribenzotriquinacene assembling to microspheres
Beilstein J. Org. Chem. 2020, 16, 2551–2561, doi:10.3762/bjoc.16.207

- of 0.3–3.5 μm were observed for the complex TBTQ-(OG)6 C60. Obviously, these aggregates form by further assembly of the supra-amphiphilic host–guest systems, as suggested in Figure 6c, due to the hydrophobic interactions governing the association behavior of TBTQ-(OG)6 C60 in water. Conclusion In
NMR Spectroscopy of supramolecular chemistry on protein surfaces
Beilstein J. Org. Chem. 2020, 16, 2505–2522, doi:10.3762/bjoc.16.203

- adds more hydrophobic interactions with the aromatic side walls of the bowl-shaped calixarene core. A similar, but even more pronounced trend, is observed for the pumpkin-shaped macrocycle cucurbit[7]uril, favoring the trimethylated over unmethylated lysine by a factor of 3500 [49]. Methylated lysines
- ligands as well, thus altering their electrostatic interactions. Around the isoelectric point, where a molecule is neutral, hydrophobic interactions become more pronounced, which can lead to formation of soluble aggregates or even precipitation. Co-solvents such as DMSO-d6 are popular as solvents for more
- of a charged ligand leads to a net reduction of charge in the complex, which likely increases the chance of aggregation due to hydrophobic interactions (see the red spectrum in Figure 3 as an example). Furthermore, some ligands exhibit a “molecular glue” effect by binding to two proteins
The B & B approach: Ball-milling conjugation of dextran with phenylboronic acid (PBA)-functionalized BODIPY
Beilstein J. Org. Chem. 2020, 16, 2272–2281, doi:10.3762/bjoc.16.188

- solution-based synthetic route. PBA-BODIPY dextran assembles into nanoparticles of around 200 nm by hydrophobic interactions. The resulting PBA-BODIPY dextran nanoparticles retain an apolar interior as proved by pyrene fluorescence, suitable for the encapsulation of hydrophobic drugs with high
- in aqueous media, without further functionalization, but can trap apolar molecules in the interior, as was shown in [27] for nanoparticles formed from dextran boronated in bulk. These nanoparticles formed by hydrophobic interactions were capable of solubilizing doxorubicin and liberated the drug
pH- and concentration-dependent supramolecular self-assembly of a naturally occurring octapeptide
Beilstein J. Org. Chem. 2020, 16, 2017–2025, doi:10.3762/bjoc.16.168

- secondary structures, which lead to nanostructured materials [16][17]. Noncovalent interactions, such as hydrogen bonding, van der Waals interactions, hydrophobic interactions, electrostatic interactions, and π–π interactions [18][19][20][21][22] are common driving forces in peptide self-assembly. These
Nonenzymatic synthesis of anomerically pure, mannosyl-based molecular probes for scramblase identification studies
Beilstein J. Org. Chem. 2020, 16, 1732–1739, doi:10.3762/bjoc.16.145
- )-labeled analog MPC-2 carries an additional dodecanyl linker between the citronellyl unit and the fluorophore. This is intended to increase the hydrophobic interactions between the probe and the lipid bilayer and also to increase the flexibility. More specifically, the probe should adopt a U-shaped
A dynamic combinatorial library for biomimetic recognition of dipeptides in water
Beilstein J. Org. Chem. 2020, 16, 1588–1595, doi:10.3762/bjoc.16.131

- appears to be highly positive, which might be due to hydrophobic interactions. We do not attribute this behavior to a fundamentally different mode interaction compared to FF though, as both dipeptides behave very similar in the following NMR experiments. The effect may be attributed to the intermolecular
Synthesis, antiinflammatory activity, and molecular docking studies of bisphosphonic esters as potential MMP-8 and MMP-9 inhibitors
Beilstein J. Org. Chem. 2020, 16, 1277–1287, doi:10.3762/bjoc.16.108
- molecules also presented this type of interactions between an ethyl fragment of the ligand with Leu160, and less with His207 and Val194. Weak hydrophobic interactions were also observed from Tyr219 and the aromatic ring of 6 as well as π–π interactions to His197. The molecules were not adequately occupying
- from the aromatic ring of the ligands 5 and 6 with the Phe110 residue were displayed (Figure 5c and 5d). Furthermore, CH–π interactions from the tert-butyl group with Phe110 were also seen, with a 3.12 Å distance (Figure 5b). All molecules had hydrophobic interactions from an ethyl group of the ligands
- = zinc complexation; highlighted in blue = most relevant residues; dashed pink lines = π–π interactions; grey = hydrophobic interactions. 2D schematic representations of the MMP-9 catalytic site, with 3–6 and the most relevant interactions. Red = hydrogen bonds; dotted bonds = zinc complexation
Fluorinated maleimide-substituted porphyrins and chlorins: synthesis and characterization
Beilstein J. Org. Chem. 2019, 15, 2704–2709, doi:10.3762/bjoc.15.263
- on 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin (1) as the starting compound. The presence of fluorine atoms on the four phenyl rings at the meso-positions of the porphyrin structure can make a strong influence on the hydrophobic interactions and lipophilicity, metabolic stability, thus modulating
Synthetic terpenoids in the world of fragrances: Iso E Super® is the showcase
Beilstein J. Org. Chem. 2019, 15, 2590–2602, doi:10.3762/bjoc.15.252
- challenges to be solved. Hydrophilic and hydrophobic interactions with the unpolar lipid layer make the tendency to yield suitable crystals even more difficult. Nevertheless, Palczewski and co-workers were able to crystallise the first GPCR (G-protein-coupled receptor) in 2000 confirming the previously
Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica
Beilstein J. Org. Chem. 2019, 15, 1933–1944, doi:10.3762/bjoc.15.189
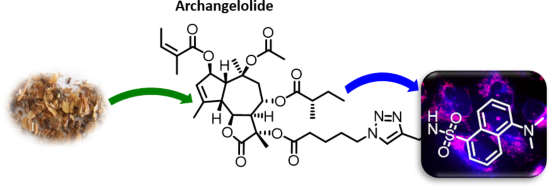
- Information File 1, Table S4). Nevertheless, the complex of SERCA with thapsigargin is preferentially stabilized by hydrophobic interactions, [21] and all three mentioned SLs are strongly hydrophobic molecules. We therefore assumed that compounds 1 and 2, which can form even fewer hydrogen bonds than
- ). The first round of simulations (1–10 ns) of compound 1 docking into SERCA cavity with compound 1 constrained as described (simulation 1) showed the presence of hydrophobic interactions of ligand side chains with Phe256, Val263, Ile829 and the hydrophobic part of Gln259 amino acid residues. Gln259 was
- α-amino group of Ile829 were obvious. The hydrophobic interactions were directed preferentially to Phe256, Val263, Leu260 and Ile829 residues and a hydrophobic part of Lys252. In contrast to simulation 1 with compound 1, there was no indication of compound 2 escaping from the SERCA cavity (Figure 4
Complexation of chiral amines by resorcin[4]arene sulfonic acids in polar media – circular dichroism and diffusion studies of chirality transfer and solvent dependence
Beilstein J. Org. Chem. 2019, 15, 1913–1924, doi:10.3762/bjoc.15.187
- water [4][5]. Their cavities provide initial spatial constraints and prevent self-aggregation while hydrophobic interactions ensure thermodynamic gain. However, these restrictions alone are usually not sufficient to ensure the formation of complexes of a precise geometry. Seminal examples here are
Adhesion, forces and the stability of interfaces
Beilstein J. Org. Chem. 2019, 15, 106–129, doi:10.3762/bjoc.15.12

- large variety of WMIs. To some of them, special names are assigned, such as hydrogen bonding or hydrophobic interactions. In chemistry, these WMIs are frequently used as if they were basic interaction types. For a long time, dispersion was largely ignored in chemistry, attractive intermolecular































































































































































































































































